Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization
Abstract
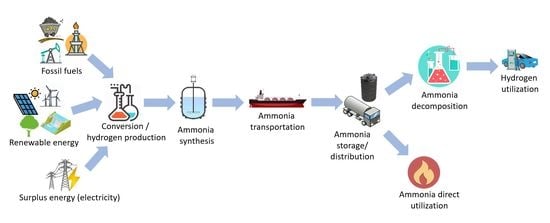
:1. Introduction
2. Characteristics of Ammonia
2.1. Physical Properties
2.2. Hydrogen Storage Performance
3. Ammonia Production
3.1. Conventional Ammonia Production (Haber–Bosch Process)
3.2. Electrochemical Processing
3.3. Thermochemical Cycle of Ammonia Production
3.4. Advanced Ammonia Production Systems
4. Ammonia Storage and Transportation
5. Ammonia Utilization
5.1. Internal Combustion Engine
5.2. Turbine-Based Power Generation
5.3. Direct Ammonia Fuel Cell
5.4. Mixing with Other Fuels
5.5. Ammonia Decomposition
6. Challenges and Recommendations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ekwurzel, B.; Boneham, J.; Dalton, M.W.; Heede, R.; Mera, R.J.; Allen, M.R.; Frumhoff, P.C. The rise in global atmospheric CO2, surface temperature, and sea level from emissions traced to major carbon producers. Clim. Chang. 2017, 144, 579–590. [Google Scholar] [CrossRef] [Green Version]
- United Nations Framework Convention on Climate Change (UNFCCC). Report of the conference of the parties on COP 21. In Proceedings of the Conference of the Parties on its Twenty-First Session, Paris, France, 30 November–13 December 2015; Volume 1, p. 1192. [Google Scholar]
- United Nations Framework Convention on Climate Change (UNFCCC). Marrakech Partnership at COP 25. Available online: https://unfccc.int/climate-action/marrakech-partnership-at-cop-25 (accessed on 2 February 2020).
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrog. Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Juangsa, F.B.; Prananto, L.A.; Mufrodi, Z.; Budiman, A.; Oda, T.; Aziz, M. Highly energy-efficient combination of dehydrogenation of methylcyclohexane and hydrogen-based power generation. Appl. Energy 2018, 226, 31–38. [Google Scholar] [CrossRef]
- Aziz, M.; Juangsa, F.B.; Kurniawan, W.; Budiman, B.A. Clean co-production of H2 and power from low rank coal. Energy 2016, 116, 489–497. [Google Scholar] [CrossRef]
- US Department of Energy Hydrogen Storage Challenges. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage-challenges (accessed on 9 April 2020).
- Zhang, Y.H.; Jia, Z.C.; Yuan, Z.M.; Yang, T.; Qi, Y.; Zhao, D.L. Development and application of hydrogen storage. J. Iron Steel Res. Int. 2015, 22, 757–770. [Google Scholar] [CrossRef]
- Chalk, S.G.; Miller, J.F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for clean energy systems. J. Power Sour. 2006, 159, 73–80. [Google Scholar] [CrossRef]
- Shukla, A.; Karmakar, S.; Biniwale, R.B. Hydrogen delivery through liquid organic hydrides: Considerations for a potential technology. Int. J. Hydrog. Energy 2012, 37, 3719–3726. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Nakaso, K.; Aoki, T.; Kitazato, Y.; Fukai, J. Effect of pressure, composition and temperature characteristics on thermal response and overall reaction rates in a metal hydride tank. Int. J. Hydrog. Energy 2011, 36, 3529–3536. [Google Scholar] [CrossRef]
- Hadjixenophontos, E.; Dematteis, E.M.; Berti, N.; Wołczyk, A.R.; Huen, P.; Brighi, M.; Le, T.T.; Santoru, A.; Payandeh, S.H.; Peru, F.; et al. A review of the MSCA ITN ECOSTORE-Novel complex metal hydrides for efficient and compact storage of renewable energy as hydrogen and electricity. Inorganics 2020, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, S.; Nishimura, M.; Harada, E. Study on introduction of CO2 free energy to Japan with liquid hydrogen. Phys. Procedia 2015, 67, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Sun, C.; Shen, S.; Zou, J.; Mao, S.S.; Yao, X. Combination of nanosizing and interfacial effect: Future perspective for designing Mg-based nanomaterials for hydrogen storage. Renew. Sustain. Energy Rev. 2015, 44, 289–303. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fang, B. Electrochemical hydrogen storage: Opportunities for fuel storage, batteries, fuel cells, and supercapacitors. Int. J. Hydrog. Energy 2017, 42, 25143–25165. [Google Scholar] [CrossRef]
- Chamoun, R.; Demirci, U.B.; Miele, P. Cyclic dehydrogenation-(Re)hydrogenation with hydrogen-storage materials: An overview. Energy Technol. 2015, 3, 100–117. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Babu, A.R.V.; Devunuri, N.; Prashanthi, Y.; Merugu, R.; Teja, A.J.R. Magnesium hydrides for hydrogen storage: A mini review. Int. J. Chemtech. Res. 2014, 6, 3451–3455. [Google Scholar]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. Chem. Sus. Chem. 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Surya-Prakash, G.K.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products-closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Ikäheimo, J.; Kiviluoma, J.; Weiss, R.; Holttinen, H. Power-to-ammonia in future north European 100% renewable power and heat system. Int. J. Hydrog. Energy 2018, 43, 17295–17308. [Google Scholar] [CrossRef]
- Fecke, M.; Garner, S.; Cox, B. Review of global regulations for anhydrous ammonia production, use, and storage. Inst. Chem. Eng. Symp. Ser. 2016, 2016, 1–11. [Google Scholar]
- Frattini, D.; Cinti, G.; Bidini, G.; Desideri, U.; Cioffi, R.; Jannelli, E. A system approach in energy evaluation of different renewable energies sources integration in ammonia production plants. Renew. Energy 2016, 99, 472–482. [Google Scholar] [CrossRef]
- Ajiwibowo, M.W.; Darmawan, A.; Aziz, M. A conceptual chemical looping combustion power system design in a power-to-gas energy storage scenario. Int. J. Hydrog. Energy 2019, 44, 9636–9642. [Google Scholar] [CrossRef]
- Crolius, S.H. NH3 Energy Implementation Conference: A Brief Report. Available online: https://www.ammoniaenergy.org/articles/nh3-energy-implementation-conference-a-brief-report/ (accessed on 5 March 2020).
- Muraki, S. Development of technologies to utilize green ammonia in energy market policies and actions toward a low carbon society. In Proceedings of the 2018 NH3 Fuel Conference, Pittsburgh, PA, USA, 1 November 2018; p. 20. [Google Scholar]
- Crolius, S.H. Great Strides in NH3 Commitment and Progress in Australia. Available online: https://www.ammoniaenergy.org/articles/great-strides-in-nh3-commitment-and-progress-in-australia/ (accessed on 27 February 2020).
- Brown, T. HIAlba-IDEA: Think Tank Launches in Scotland to Deploy CSIRO Technology, Become Green Energy Exporter. Available online: https://www.ammoniaenergy.org/articles/hialba-idea-think-tank-launches-in-scotland-to-deploy-csiro-technology-become-green-energy-exporter/ (accessed on 27 February 2020).
- Bennani, Y.; Bennani, Y.; Perl, A.; Patil, A.; van Someren, C.E.J.; Heijne, L.J.M.; van Steenis, M.; Patil, A.; van Someren, C.E.J.; Heijne, L.J.M.; et al. Power-to-Ammonia: Rethinking the Role of Ammonia-From a Value Product to a Flexible Energy Carrier; Hanzehogeschool Groningen: Groningen, The Netherlands, 2016; pp. 1–110. [Google Scholar]
- Zamfirescu, C.; Dincer, I. Ammonia as a green fuel and hydrogen source for vehicular applications. Fuel Process. Technol. 2009, 90, 729–737. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrog. Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- National Library of Medicine Ammonia. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia (accessed on 28 April 2020).
- National Institute of Standard and Technology Ammonia. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C7664417 (accessed on 28 April 2020).
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrog. Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Metkemeijer, R.; Achard, P. Comparison of ammonia and methanol applied indirectly in a hydrogen fuel cell. Int. J. Hydrog. Energy 1994, 19, 535–542. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; Jongh, P.E.; de Chen, P.; et al. Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress. Int. J. Hydrog. Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Cross-ministerial Strategic Innovation Promotion Program (SIP) Energy Carriers 2016. Available online: https://www.jst.go.jp/sip/pdf/SIP_energycarriers2015_en.pdf (accessed on 28 April 2020).
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Gong, J. Alternative strategies toward sustainable ammonia synthesis. Trans. Tianjin Univ. 2020, 26, 67–91. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Yuhas, B.D.; Margulies, E.A.; Zhang, Y.; Shim, Y.; Wasielewski, M.R.; Kanatzidis, M.G. Photochemical nitrogen conversion to ammonia in ambient conditions with femos-chalcogels. J. Am. Chem. Soc. 2015, 137, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Klinsrisuk, S.; Tao, S.; Irvine, J.T.S. 18-Membrane reactors for ammonia production. In Membrane Reactors for Energy Applications and Basic Chemical Production; Basile, A., Paola, L., Di, l., Hai, F., Piemonte, V., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2015; pp. 543–563. ISBN 978-1-78242-223-5. [Google Scholar]
- Rafiqul, I.; Weber, C.; Lehmann, B.; Voss, A. Energy efficiency improvements in ammonia production-perspectives and uncertainties. Energy 2005, 30, 2487–2504. [Google Scholar] [CrossRef]
- Wang, L.; Xia, M.; Wang, H.; Huang, K.; Qian, C.; Maravelias, C.T.; Ozin, G.A. Greening ammonia toward the solar ammonia refinery. Joule 2018, 2, 1055–1074. [Google Scholar] [CrossRef]
- Avery, W.H. A role for ammonia in the hydrogen economy. Int. J. Hydrog. Energy 1988, 13, 761–773. [Google Scholar] [CrossRef]
- Cheema, I.I.; Krewer, U. Operating envelope of Haber-Bosch process design for power-to-ammonia. RSC Adv. 2018, 8, 34926–34936. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.R.; Klosek, J. A review of air separation technologies and their.pdf. Fuel Process. Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Potential for improving the energy efficiency of cryogenic air separation unit (ASU) using binary heat recovery cycles. Appl. Eng. 2015, 81, 223–231. [Google Scholar] [CrossRef]
- Gilbert, P.; Thornley, P. Energy and carbon balance of ammonia production from biomass gasification. In Proceedings of the Bio-Ten Conference, Birmingham, UK, 20–22 September 2010; pp. 1–9. [Google Scholar]
- Wang, Q.; Guo, J.; Chen, P. Recent progress towards mild-condition ammonia synthesis. J. Energy Chem. 2019, 36, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Siporin, S.E.; Davis, R.J. Use of kinetic models to explore the role of base promoters on Ru/MgO ammonia synthesis catalysts. J. Catal. 2004, 225, 359–368. [Google Scholar] [CrossRef]
- Brown, D.E.; Edmonds, T.; Joyner, R.W.; McCarroll, J.J.; Tennison, S.R. The genesis and development of the commercial BP doubly promoted catalyst for ammonia synthesis. Catal. Lett. 2014, 144, 545–552. [Google Scholar] [CrossRef]
- Bielawa, H.; Hinrichsen, O.; Birkner, A.; Muhler, M. The ammonia-synthesis catalyst of the next generation: Barium-promoted oxide-supported ruthenium. Angew. Chem. Int. Ed. 2001, 40, 1061–1063. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Ishikawa, H.; Yamagata, K.; Nakao, T.; Tada, T.; Matsuishi, S.; Yokoyama, T.; Hara, M.; Hosono, H. Essential role of hydride ion in ruthenium-based ammonia synthesis catalysts. Chem. Sci. 2016, 7, 4036–4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Vojvodic, A.; Medford, A.J.; Studt, F.; Abild-Pedersen, F.; Khan, T.S.; Bligaard, T.; Nørskov, J.K. Exploring the limits: A low-pressure, low-temperature Haber-Bosch process. Chem. Phys. Lett. 2014, 598, 108–112. [Google Scholar] [CrossRef]
- Kawamura, F.; Taniguchi, T. Synthesis of ammonia using sodium melt. Sci. Rep. 2017, 7, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipman, T.; Shah, N. Ammonia as an Alternative Energy Storage Medium for Hydrogen Fuel Cells: Scientific and Technical Review for Near-Term Stationary Power Demonstration Projects; Final Report; University of California: Berkeley, CA, USA, 2017. [Google Scholar]
- Tsuneto, A.; Kudo, A.; Sakata, T. Lithium-mediated electrochemical reduction of high pressure N2 to NH3. J. Electroanal. Chem. 1994, 367, 183–188. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [Green Version]
- Kamer, P.C.J.; Vogt, D.; Thybaut, J. Contemporary Catalysis; The Royal Society of Chemistry: London, UK, 2017; ISBN 978-1-84973-990-0. [Google Scholar]
- Murakami, T.; Nishikiori, T.; Nohira, T.; Ito, Y. Electrolytic synthesis of ammonia in molten salts under atmospheric pressure. J. Am. Chem. Soc. 2003, 125, 334–335. [Google Scholar] [CrossRef]
- Amar, I.A.; Lan, R.; Petit, C.T.G.; Arrighi, V.; Tao, S. Electrochemical synthesis of ammonia based on a carbonate-oxide composite electrolyte. Solid State Ion. 2011, 182, 133–138. [Google Scholar] [CrossRef]
- Iwahara, H. Technological challenges in the application of proton conducting ceramics. Solid State Ion. 1995, 77, 289–298. [Google Scholar] [CrossRef]
- Soloveichik, G. Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat. Catal. 2019, 2, 377–380. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Halmann, M.; Steinfeld, A. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 1. Thermodynamic, environmental, and economic analyses. Ind. Eng. Chem. Res. 2007, 46, 2042–2046. [Google Scholar]
- Gálvez, M.E.; Frei, A.; Halmann, M.; Steinfeld, A. Ammonia production via a two-step Al2O3/AIN thermochemical cycle 2. Kinetic analysis. Ind. Eng. Chem. Res. 2007, 46, 2047–2053. [Google Scholar] [CrossRef]
- Juangsa, F.B.; Aziz, M. Integrated system of thermochemical cycle of ammonia, nitrogen production, and power generation. Int. J. Hydrog. Energy 2019, 44, 17525–17534. [Google Scholar] [CrossRef]
- Cinti, G.; Frattini, D.; Jannelli, E.; Desideri, U.; Bidini, G. Coupling solid oxide electrolyser (SOE) and ammonia production plant. Appl. Energy 2017, 192, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.; Putranto, A.; Biddinika, M.K.; Wijayanta, A.T. Energy-saving combination of N2 production, NH3 synthesis, and power generation. Int. J. Hydrog. Energy 2017, 42, 27174–27183. [Google Scholar] [CrossRef]
- Darmawan, A.; Ajiwibowo, M.W.; Yoshikawa, K.; Aziz, M.; Tokimatsu, K. Energy-efficient recovery of black liquor through gasification and syngas chemical looping. Appl. Energy 2018, 219, 290–298. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Zaini, I.N.; Amin, M.; Sasongko, D.; Aziz, M. Microalgae-based coproduction of ammonia and power employing chemical looping process. Chem. Eng. Res. Des. 2019, 146, 311–323. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Aziz, M. Ammonia production from algae via integrated hydrothermal gasification, chemical looping, N2 production, and NH3 synthesis. Energy 2019, 174, 331–338. [Google Scholar] [CrossRef]
- Ajiwibowo, M.W.; Darmawan, A.; Aziz, M. Towards clean palm oil processing: Integrated ammonia production from empty fruit bunch and palm oil effluent. J. Clean. Prod. 2019, 236, 117680. [Google Scholar] [CrossRef]
- National Institute of Standard and Technology Thermophysical Properties of Fluid Systems. Available online: http://webbook.nist.gov/chemistry/fluid/ (accessed on 2 March 2020).
- Christensen, C.H.; Sørensen, R.Z.; Johannessen, T.; Quaade, U.J.; Honkala, K.; Elmøe, T.D.; Køhler, R.; Nørskov, J.K. Metal ammine complexes for hydrogen storage. J. Mater. Chem. 2005, 15, 4106–4108. [Google Scholar] [CrossRef]
- Jacobsen, H.S.; Hansen, H.A.; Andreasen, J.W.; Shi, Q.; Andreasen, A.; Feidenhans’l, R.; Nielsen, M.M.; Ståhl, K.; Vegge, T. Nanoscale structural characterization of Mg(NH3)6Cl2 during NH3 desorption: An in situ small angle X-ray scattering study. Chem. Phys. Lett. 2007, 441, 255–260. [Google Scholar] [CrossRef]
- EasyChem Industrial Uses of Ammonia. Available online: https://easychem.com.au/monitoring-and-management/maximising-production/industrial-uses-of-ammonia/ (accessed on 4 March 2020).
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Osaka, Y.; Zeng, T. Numerical study on effect of oxygen content in combustion air on ammonia combustion. Energy 2015, 93, 2053–2068. [Google Scholar] [CrossRef]
- Kroch, E. Ammonia-a fuel for motor buses. J. Inst. Pet. 1945, 31, 213–223. [Google Scholar]
- Mario, Z. Device for Operating Internal Combustion Engines with Mixtures of Ammonia, Hydrogen and Nitrogen Preapred from Ammonia. U.S. Patent US2140254A, 13 December 1938. [Google Scholar]
- NASA. NASA Armstrong Fact Sheet: X-15 Hypersonic Research Program. Available online: https://www.nasa.gov/centers/armstrong/news/FactSheets/FS-052-DFRC.html (accessed on 10 March 2020).
- Starkman, E.S.; Newhall, H.K.; Sutton, R.; Maguire, T.; Farbar, L. Ammonia as a spark ignition engine fuel: Theory and application. SAE Trans. 1967, 75, 765–784. [Google Scholar]
- Pearsall, T.J.; Garabedian, C.G. Combustion of anhydrous ammonia in diesel engines. SAE Trans. 1968, 76, 3213–3221. [Google Scholar]
- Cornelius, W.; Huellmantel, L.W.; Mitchell, H.R. Ammonia as an Engine Fuel; Society of Automotive Engineers: Warrendale, PA, USA, 1965. [Google Scholar]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia-methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Leo Willi, M. Power System having an Ammonia Fuelled Engine. U.S. Patent US20100019506A1, 28 January 2010. [Google Scholar]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.C. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Boretti, A. Novel dual fuel diesel-ammonia combustion system in advanced TDI engines. Int. J. Hydrog. Energy 2017, 42, 7071–7076. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R. Analysis of the behaviour of a 4-stroke Si engine fuelled with ammonia and hydrogen. Int. J. Hydrog. Energy 2013, 38, 1607–1615. [Google Scholar] [CrossRef]
- Faehn, D.; Bull, M.G.; Shekleton, J.R. Experimental investigation of ammonia as a turbine engine fuel. SAE Tech. Pap. 1966, 660769. [Google Scholar]
- Valera-Medina, A.; Pugh, D.G.; Marsh, P.; Bulat, G.; Bowen, P. Preliminary study on lean premixed combustion of ammonia-hydrogen for swirling gas turbine combustors. Int. J. Hydrog. Energy 2017, 42, 24495–24503. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]
- Karabeyoglu, A.; Evans, B. Fuel conditioning system for ammonia-fired power plants. In Proceedings of the 9th Annual NH3 Fuel Association Conference, San Antonio, TX, USA, 1 October 2012. [Google Scholar]
- Meyer, T.; Kumar, P.; Li, M.; Redfern, K.; Diaz, D. Ammonia combustion with near-zero pollutant emissions. In Proceedings of the 7th Annual NH3 Fuel Conference, Romulus, MI, USA, 26–28 September 2010. [Google Scholar]
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Nagai, Y. Study on using hydrogen and ammonia as fuels: Combustion characteristics and NOxformation. Int. J. Energy Res. 2014, 38, 1214–1223. [Google Scholar] [CrossRef]
- Newhall, H.K.; Starkman, E.S. Theoretical performance of ammonia as a gas turbine fuel. SAE Tech. Pap. 1966, 660768. [Google Scholar]
- Lee, D.; Song, H.H. Development of combustion strategy for the internal combustion engine fueled by ammonia and its operating characteristics. J. Mech. Sci. Technol. 2018, 32, 1905–1925. [Google Scholar] [CrossRef]
- Keller, M.; Koshi, M.; Otomo, J.; Iwasaki, H.; Mitsumori, T.; Yamada, K. Thermodynamic evaluation of an ammonia-fueled combined-cycle gas turbine process operated under fuel-rich conditions. Energy 2020, 194, 116894. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3-air and NH3-CH4-air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Goshome, K.; Yamada, T.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. High compressed hydrogen production via direct electrolysis of liquid ammonia. Int. J. Hydrog. Energy 2016, 41, 14529–14534. [Google Scholar] [CrossRef] [Green Version]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. An improved electrochemical model for the NH3 fed proton conducting solid oxide fuel cells at intermediate temperatures. J. Power Sources 2008, 185, 233–240. [Google Scholar] [CrossRef]
- De Geeter, E.; Mangan, M.; Spaepen, S.; Stinissen, W.; Vennekens, G. Alkaline fuel cells for road traction. J. Power Sour. 1999, 80, 207–212. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Munnings, C.; Dolan, M. Ammonia as a renewable energy transportation media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- McLean, G.F.; Niet, T.; Prince-Richard, S.; Djilali, N. An assessment of alkaline fuel cell technology. Int. J. Hydrog. Energy 2002, 27, 507–526. [Google Scholar] [CrossRef]
- Suzuki, S.; Muroyama, H.; Matsui, T.; Eguchi, K. Fundamental studies on direct ammonia fuel cell employing anion exchange membrane. J. Power Sour. 2012, 208, 257–262. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Direct ammonia alkaline anion-exchange membrane fuel cells. Electrochem. Solid State Lett. 2010, 13. [Google Scholar] [CrossRef]
- Lee, K.R.; Song, D.; Park, S.B.; Han, J.I. A direct ammonium carbonate fuel cell with an anion exchange membrane. RSC Adv. 2014, 4, 5638–5641. [Google Scholar] [CrossRef]
- Yao, K.; Cheng, Y.F. Electrodeposited Ni–Pt binary alloys as electrocatalysts for oxidation of ammonia. J. Power Sour. 2007, 173, 96–101. [Google Scholar] [CrossRef]
- Lomocso, T.L.; Baranova, E.A. Electrochemical oxidation of ammonia on carbon-supported bi-metallic PtM (M = Ir, Pd, SnOx) nanoparticles. Electrochim. Acta 2011, 56, 8551–8558. [Google Scholar] [CrossRef]
- Endo, K.; Nakamura, K.; Katayama, Y.; Miura, T. Pt–Me (Me = Ir, Ru, Ni) binary alloys as an ammonia oxidation anode. Electrochim. Acta 2004, 49, 2503–2509. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Farr, R.D.; Vayenas, C.G. Ammonia high temperature solid electrolyte fuel cell. J. Electrochem. Soc. 1980, 127, 1478–1483. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Farr, R.D. Cogeneration of electric energy and nitric oxide. Science 1980, 208, 593–594. [Google Scholar] [CrossRef]
- Wojcik, A.; Middleton, H.; Damopoulos, I.; Van Herle, J. Ammonia as a fuel in solid oxide fuel cells. J. Power Sour. 2003, 118, 342–348. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. Thermodynamic analysis of ammonia fed solid oxide fuel cells: Comparison between proton-conducting electrolyte and oxygen ion-conducting electrolyte. J. Power Sour. 2008, 183, 682–686. [Google Scholar] [CrossRef]
- Li, Z.; Liu, R.; Wang, J.; Xu, Z.; Xie, Y.; Wang, B. Preparation of double-doped BaCeO3 and its application in the synthesis of ammonia at atmospheric pressure. Sci. Technol. Adv. Mater. 2007, 8, 566–570. [Google Scholar] [CrossRef]
- Meng, G.; Jiang, C.; Ma, J.; Ma, Q.; Liu, X. Comparative study on the performance of a SDC-based SOFC fueled by ammonia and hydrogen. J. Power Sour. 2007, 173, 189–193. [Google Scholar] [CrossRef]
- Maffei, N.; Pelletier, L.; Charland, J.P.; McFarlan, A. An intermediate temperature direct ammonia fuel cell using a proton conducting electrolyte. J. Power Sour. 2005, 140, 264–267. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hayakawa, A.; Kitagawa, Y.; Kunkuma, A.S.K.D.; Kudo, T.; Kobayashi, H. Laminar burning velocity and markstein length of ammonia/hydrogen/air premixed flames at elevated pressures. Int. J. Hydrog. Energy 2015, 40, 9570–9578. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, J.H.; Park, J.H.; Kwon, O.C. Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production. Int. J. Hydrog. Energy 2010, 35, 1054–1064. [Google Scholar] [CrossRef]
- Kumar, P.; Meyer, T.R. Experimental and modeling study of chemical-kinetics mechanisms for H 2-NH3-air mixtures in laminar premixed jet flames. Fuel 2013, 108, 166–176. [Google Scholar] [CrossRef]
- Henshaw, P.F.; D’Andrea, T.; Mann, K.R.C.; Ting, D.S.K. Premixed ammonia-methane-air combustion. Combust. Sci. Technol. 2005, 177, 2151–2170. [Google Scholar] [CrossRef]
- Zietz, U.; Baumgärtel, G. The laminar flame speeds of propane-ammonia-air mixtures. Combust. Flame 1969, 13, 329–330. [Google Scholar] [CrossRef]
- Bockhorn, H.; Fetting, F.; Mende, J.C. The laminar flame velocities of propane/ammonia mixtures. Combust. Flame 1972, 18, 471–473. [Google Scholar] [CrossRef]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; Van Der Gryp, P.; Bessarabov, D.G. Reactor technology options for distributed hydrogen generation via ammonia decomposition: A review. Int. J. Hydrog. Energy 2013, 38, 14968–14991. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrog. Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Ganley, J.C.; Thomas, F.S.; Seebauer, E.G.; Masel, R.I. A priori catalytic activity correlations: The difficult case of hydrogen production from ammonia. Catal. Lett. 2004, 96, 117–122. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A Gen. 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Boisen, A.; Dahl, S.; Nørskov, J.K.; Christensen, C.H. Why the optimal ammonia synthesis catalyst is not the optimal ammonia decomposition catalyst. J. Catal. 2005, 230, 309–312. [Google Scholar] [CrossRef]
- Bligaard, T.; Nørskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.H.; Sehested, J. The brønsted-evans-polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Wang, S.J.; Yin, S.F.; Li, L.; Xu, B.Q.; Ng, C.F.; Au, C.T. Investigation on modification of Ru/CNTs catalyst for the generation of COx-free hydrogen from ammonia. Appl. Catal. B Environ. 2004, 52, 287–299. [Google Scholar] [CrossRef]
- Hansgen, D.A.; Vlachos, D.G.; Chen, J.G. Using first principles to predict bimetallic catalysts for the ammonia decomposition reaction. Nat. Chem. 2010, 2, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-K.; Ji, W.-J.; Zhao, J.; Wang, S.-J.; Au, C.-T. Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. J. Catal. 2005, 236, 181–189. [Google Scholar] [CrossRef]
- Bajus, S.; Agel, F.; Kusche, M.; Ní Bhriain, N.; Wasserscheid, P. Alkali hydroxide-modified Ru/γ-Al2O3 catalysts for ammonia decomposition. Appl. Catal. A Gen. 2016, 510, 189–195. [Google Scholar] [CrossRef]
- Yin, S.-F.; Zhang, Q.-H.; Xu, B.-Q.; Zhu, W.-X.; Ng, C.-F.; Au, C.-T. Investigation on the catalysis of COx-free hydrogen generation from ammonia. J. Catal. 2004, 224, 384–396. [Google Scholar] [CrossRef]
- Yin, S.-F.; Xu, B.-Q.; Ng, C.-F.; Au, C.-T. Nano Ru/CNTs: A highly active and stable catalyst for the generation of COx-free hydrogen in ammonia decomposition. Appl. Catal. B Environ. 2004, 48, 237–241. [Google Scholar] [CrossRef]
- U.S. Department of Energy; Ohi, J.M.; Vanderborgh, N.; Voecks, G. Hydrogen fuel quality specifications for polymer electrolyte fuel cells in road vehicles. Saf. Codes Stand. Progr. 2016, 1–72. [Google Scholar]
- Miyaoka, H.; Miyaoka, H.; Ichikawa, T.; Ichikawa, T.; Kojima, Y. Highly purified hydrogen production from ammonia for PEM fuel cell. Int. J. Hydrog. Energy 2018, 43, 14486–14492. [Google Scholar] [CrossRef]
- Van Hassel, B.A.; Karra, J.R.; Santana, J.; Saita, S.; Murray, A.; Goberman, D.; Chahine, R.; Cossement, D. Ammonia sorbent development for on-board H2 purification. Sep. Purif. Technol. 2015, 142, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Dolan, M.D.; Viano, D.M.; Langley, M.J.; Lamb, K.E. Tubular vanadium membranes for hydrogen purification. J. Memb. Sci. 2018, 549, 306–311. [Google Scholar] [CrossRef]
- Bockris, J.O.M. The hydrogen economy: Its history. Int. J. Hydrog. Energy 2013, 38, 2579–2588. [Google Scholar] [CrossRef]
- Moliner, R.; Lázaro, M.J.; Suelves, I. Analysis of the strategies for bridging the gap towards the Hydrogen Economy. Int. J. Hydrog. Energy 2016, 41, 19500–19508. [Google Scholar] [CrossRef]
- Nejat Veziroglu, T. Conversion to hydrogen economy. Energy Procedia 2012, 29, 654–656. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F. Hydrogen energy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 1043–1056. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Hydrogen Energy and Fuel Cells: A Vision of Our Future; European Commission: Luxembourg, 2003. [Google Scholar]
- Hydrogen and Fuel Cells Strategy Office; Advanced Energy Systems and Structure Division; Energy Conservation and Renewable Energy Department. Basic Hydrogen Strategy; Trade and Industry: Tokyo, Japan, 2017; pp. 1–37.
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrog. Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Mizuno, Y.; Ishimoto, Y.; Sakai, S.; Sakata, K. Economic analysis on international hydrogen energy career supply chains. J. Jpn. Soc. Energy Resour. 2017, 38, 11–17. [Google Scholar]
- Worrell, E.; Price, L.; Neelis, M.; Galitsky, C.; Nan, Z. World Best Practice Energy Intensity Values for Selected Industrial Sectors; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2007; p. 51. [Google Scholar]
- Abashar, M.E.E. Ultra-clean hydrogen production by ammonia decomposition. J. King Saud Univ. Eng. Sci. 2018, 30, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Ammonia as a suitable fuel for fuel cells. Front. Energy Res. 2014, 2, 3–6. [Google Scholar] [CrossRef] [Green Version]






| Properties | Unit | Value |
|---|---|---|
| Molar mass | g/mol | 17.031 |
| Density at STP | kg/m3 | 0.769 |
| Melting point | °C | −77.73 |
| Boiling point at 100 kPa | °C | −33.4 |
| Vapor pressure at 20 °C | kPa | 858 |
| Heat of evaporation | MJ/kg | 1.371 |
| Auto ignition temperature | °C | 650 |
| Critical temperature | °C | 132.4 |
| Critical pressure | MPa | 11.28 |
| Viscosity at 25 °C | µPa·s | 10.07 |
| Heat capacity at constant pressure (101.325 kPa, 15 °C) | kJ/mol·°C | 0.037 |
| Heat capacity at constant volume (101.325 kPa, 15 °C) | kJ/mol·°C | 0.028 |
| Heat of combustion | MJ/L | 11.2 |
| Thermal conductivity | mW/m·°C | 22.19 |
| Critical density | g/mL | 0.24 |
| Condensation pressure at 25 °C | MPa | 0.99 |
| Flammability limit (equivalence ratio) | - | 0.63–1.4 |
| Adiabatic flame temperature | °C | 1800 |
| Max. laminar burning velocity | m/s | 0.07 |
| Properties | Unit | Compressed Hydrogen | Liquid Hydrogen | Methanol | Liquid Ammonia |
|---|---|---|---|---|---|
| Storage method | - | Compression | Liquefaction | Ambient | Liquefaction |
| Temperature | °C | 25 (room) | −252.9 | 25 (room) | 25 (room) |
| Storage pressure | MPa | 69 | 0.1 | 0.1 | 0.99 |
| Density | kg/m3 | 39 | 70.8 | 792 | 600 |
| Explosive limit in air | %vol | 4–75 | 4–75 | 6.7–36 | 15–28 |
| Gravimetric energy density (LHV) | MJ/kg | 120 | 120 | 20.1 | 18.6 |
| Volumetric energy density (LHV) | MJ/L | 4.5 | 8.49 | 15.8 | 12.7 |
| Gravimetric hydrogen content | wt% | 100 | 100 | 12.5 | 17.8 |
| Volumetric hydrogen content | kg-H2/m3 | 42.2 | 70.8 | 99 | 121 |
| Hydrogen release | - | Pressure release | Evaporation | Catalytic decomposition T > 200 °C | Catalytic decomposition T > 400 °C |
| Energy to extract hydrogen | kJ/mol-H2 | - | 0.907 | 16.3 | 30.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. https://doi.org/10.3390/en13123062
Aziz M, Wijayanta AT, Nandiyanto ABD. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies. 2020; 13(12):3062. https://doi.org/10.3390/en13123062
Chicago/Turabian StyleAziz, Muhammad, Agung Tri Wijayanta, and Asep Bayu Dani Nandiyanto. 2020. "Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization" Energies 13, no. 12: 3062. https://doi.org/10.3390/en13123062