Abstract
Introduction: Pharmacogenomics (PGx) reduces the need for ‘trial-and-error’ prescribing and the chances of adverse reactions, and improves patient outcomes. With the cost of PGx testing falling rapidly, in line with the cost of other testing within the NHS, it is already being deployed by community pharmacists outside the UK.
Aim: To learn from experiences of PGx delivery in community pharmacies in other countries to inform the set up and design of future UK pharmacy services.
Method: A five-stage scoping review methodological framework was deployed. The research question was identified and the relevant studies were selected from databases, using the search terms ‘pharmacogenomics’ OR ‘pharmacogenetics’ AND ‘community pharmacy’. A data-extraction tool was used to collect the data, which was subsequently charted into categories, including barriers, enablers, patient-orientated outcomes and recommendations for future research. The results were then collated, summarised and reported.
Results: From the 15 papers reviewed, it was noted that community pharmacy-based PGx services are becoming increasingly widespread, having been implemented in the United States, Canada, the Netherlands and Cyprus. Enablers for implementation of a PGx testing service in a community pharmacy setting included patient interest, pharmacist willingness and confidence to deliver the service, the service being comparable to existing pharmacy services (e.g. vaccination programmes) and prescriber acceptance of the results. Barriers included education and training of pharmacists, access to appropriate clinical resources, lack of patient-friendly resources and time capacity.
Conclusion: Community pharmacy-led PGx services have been reported in several different countries. For such services to work well, they need patient interest, pharmacist engagement and training, available supporting information for pharmacists and prescriber acceptance of recommendations for any changes to patient prescriptions.
Key words: Barriers, community pharmacy, enablers, pharmacogenomics, testing service
Introduction
Medicines are the most common intervention in healthcare. More than 1 billion prescriptions are dispensed in community pharmacy annually in England, at a cost of over £9bn[1]. However, medicines can be ineffective in some people for the indicated diseases and can also cause adverse drug reactions in a significant number; one explanation for this therapeutic failure is drug–gene interaction[2].
Adverse drug reactions are believed to account for 6.5% of all hospital admissions in England, with an annual cost to the NHS of £466m[3]. Patients are also less likely to take medicines that are ineffective, which can frequently occur because doctors use a ‘trial-and-error’ approach to prescribing to identify the most effective treatment[4]. This also creates significant costs for the NHS.
Pharmacogenomics
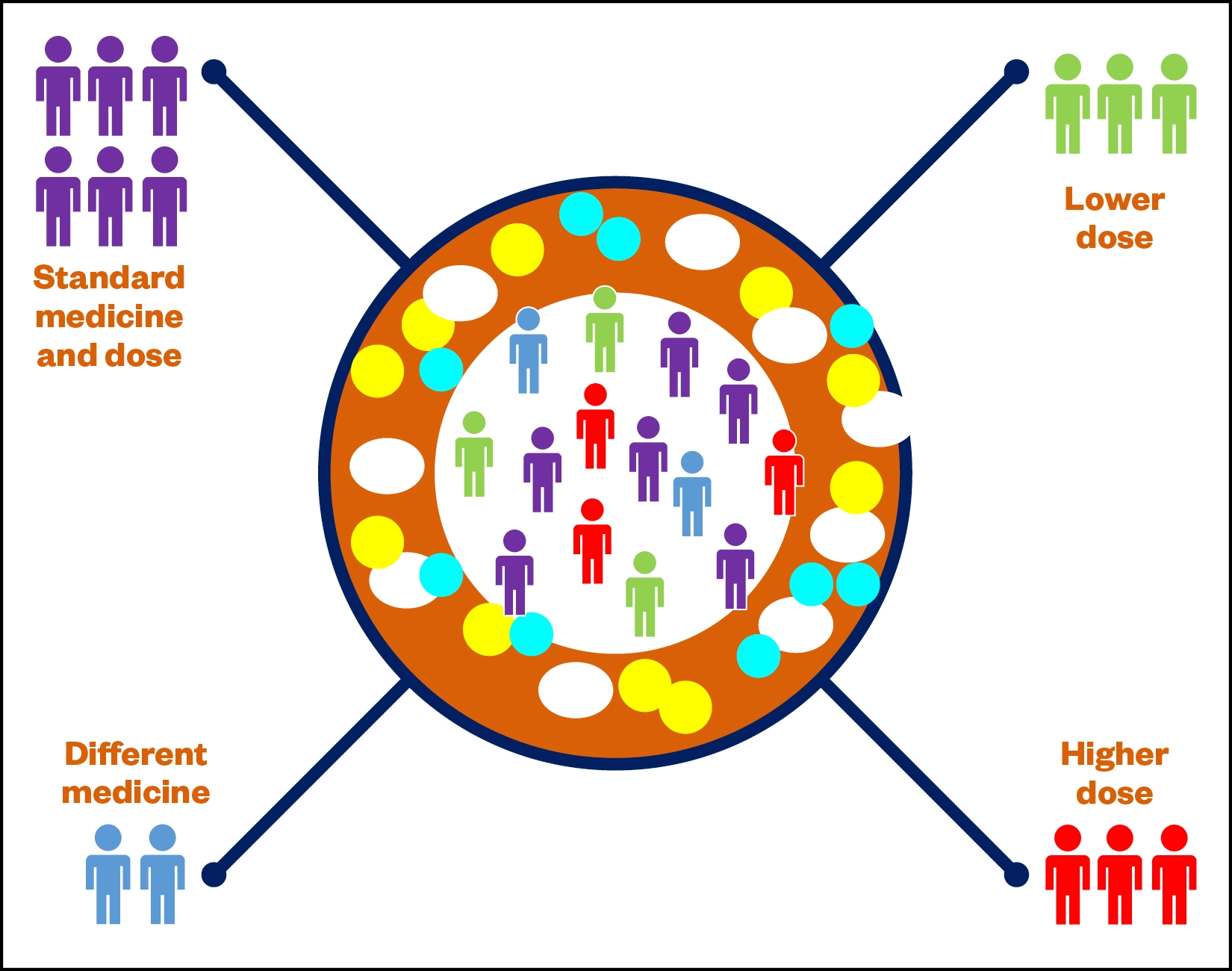
Pharmacogenomics (PGx) is a specialism of personalised medicine that uses genomic information from an individual’s DNA to predict their response to drugs[4]. Supporting the delivery of personalised medicine, PGx can be used to reduce the likelihood of adverse drug events and increase the likelihood of the best therapy and dose being selected first time[5]. PGx can move medicine and pharmacy away from the traditional ‘one-size-fits-all’ approach to one in which medicines and doses are tailored to the individual. Healthcare professionals in Norway can already test patients for 150 drugs using PGx testing in routine care[6]. There are two essential elements of PGx: pharmacokinetics and pharmacodynamics. Pharmacokinetics is the study of how patients metabolise and eliminate drugs at different rates, resulting in some people requiring a lower dose, some requiring a higher dose and others requiring a different medicine altogether (Figure 1).
Pharmacodynamics explains how patient response to the drug is altered owing to genetic differences; for example, changes in the responsiveness of the drug target[5]. Traditionally, patients have been treated with medicines based on their personal characteristics (but assuming that they are ‘average’) such as age, weight, co-medication, pregnancy, hepatic and renal disease[6]. In practice, when a prescriber or pharmacist says ‘this is the best medicine for you’, they mean ‘we know that this is the most appropriate medicine for most people, and we know this dose is the most likely to be safe’.
Community pharmacy
There are 11,400 community pharmacies in England that are less than a 20-minute walk from home for 89% of people[6]. Community pharmacists are often the last healthcare professional to see a patient before they start a new medicine, and have expertise in pharmacology and access to patient records, meaning they are ideally positioned to provide a PGx testing service. The new NHS genomics medicine service is currently being integrated into routine care, “matching people to the most effective medications and interventions, reducing the likelihood of an adverse drug reaction”[7].
A form of testing particularly amenable to the community pharmacy setting involves taking a DNA cheek swab and sending it to a testing laboratory; upon receipt of the results, a pharmacist can then review the patient’s medications and make recommendations to the prescriber to amend their prescription. It has already been suggested that offering PGx at the point of medicine initiation may be an appropriate role for community pharmacists, who can both reassure the patient about genomic testing and explain the implications of test results for current or future medicines use[5].
As no systematic reviews considering PGx introduction in community pharmacies were identified, the aim of this scoping study is to identify what research has been conducted globally in relation to PGx testing in a community pharmacy setting and to consider the implications of that research for the design of any future community pharmacy-based PGx services.
Methods
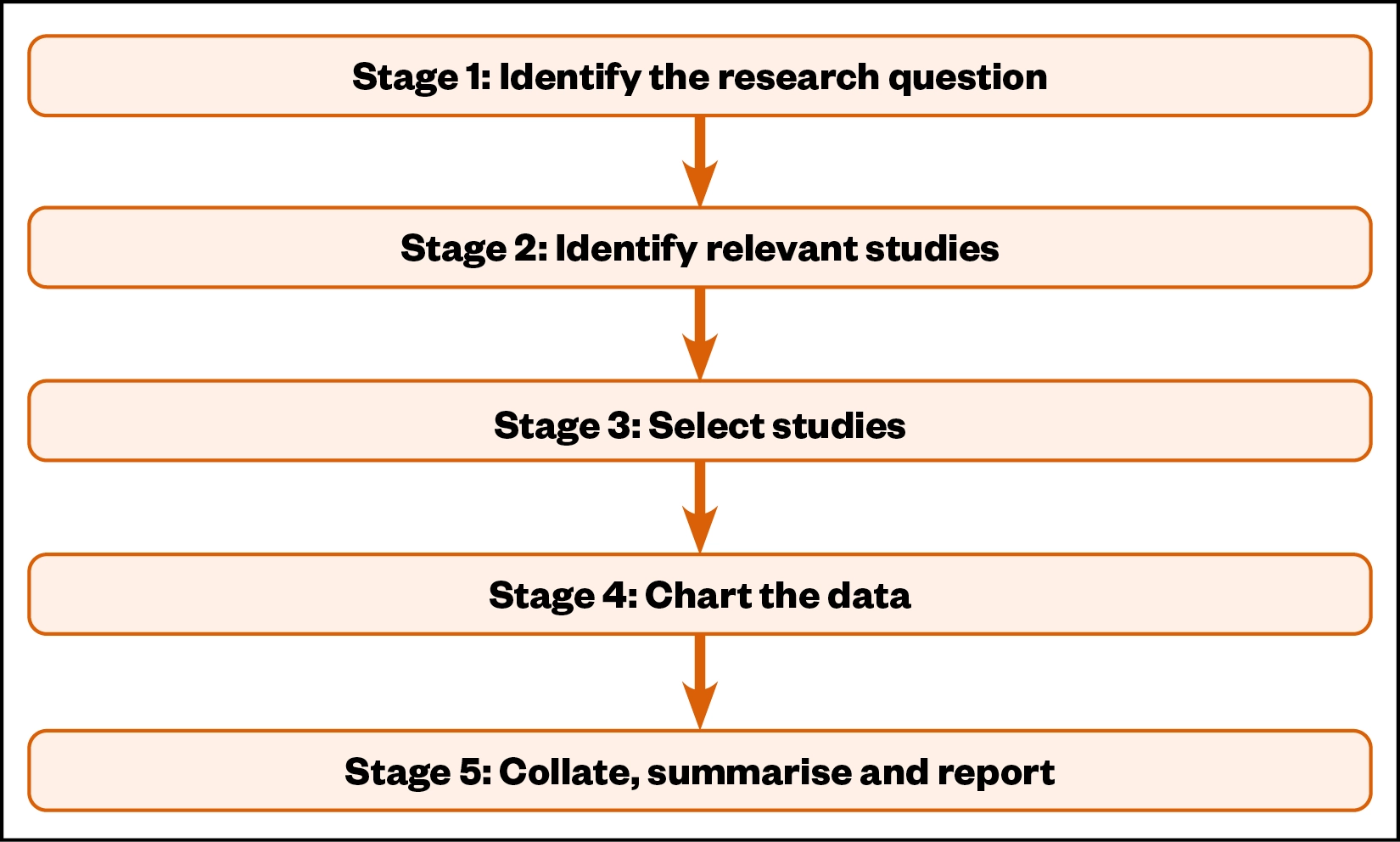
The scoping review methodological framework recommended by Arksey and O’Malley was used as it supports the review of the literature to identify concepts, evidence, theories and gaps in research, and consists of five methodological stages (see Figure 2)[8,9].
The research question
The research question was: what are the barriers and enablers to PGx service implementation in the community pharmacy context?
Identifying relevant studies
This scoping study was conducted in January 2021, with no date limits on eligibility for inclusion. Relevant studies were identified using the PubMed, Embase and Web of Science databases. Initial search terms included a combination of free-text terms and medical subject heading terms, including ‘pharmacogenomics’ OR ‘pharmacogenetics’ AND ‘community pharmacy’, with the terms ‘training’, ‘feasibility, ‘perceptions’, ‘barriers’, ‘enablers’ and ‘implementation’ used to refine the results.
Study selection
Inclusion criteria:
- Focused on the delivery of PGx in community pharmacy;
- English language;
- Includes empirical data.
Exclusion criteria:
- Conference proceedings.
Titles, abstracts and papers were screened by the researcher for inclusion at each stage.
Data extraction
The papers for review were then stored using EndNote, a software tool for publishing and managing bibliographies, citations and references. Using a devised data extraction tool, relevant details from the papers were noted and included:
- Aim of study;
- Research question;
- Method of data collection;
- Type and number of participants and settings;
- Method of participant recruitment;
- Reason for the study;
- Results and outcomes;
- Identified enablers or barriers for setting up or implementing a PGx testing service;
- Author recommendations for future research.
Finally, to inform any future training that may be required, the knowledge gaps that have been identified in the papers were also extracted.
Data analysis
The reasons reported for conducting the study were recorded in Microsoft Excel. Data was synthesised, and the barriers and enablers were collated and charted to identify themes and issues.
Ethics approval
Ethical approval was not required for a scoping review.
Results
Figure 3 summarises the scoping review process. A total of 15 papers were identified for inclusion.
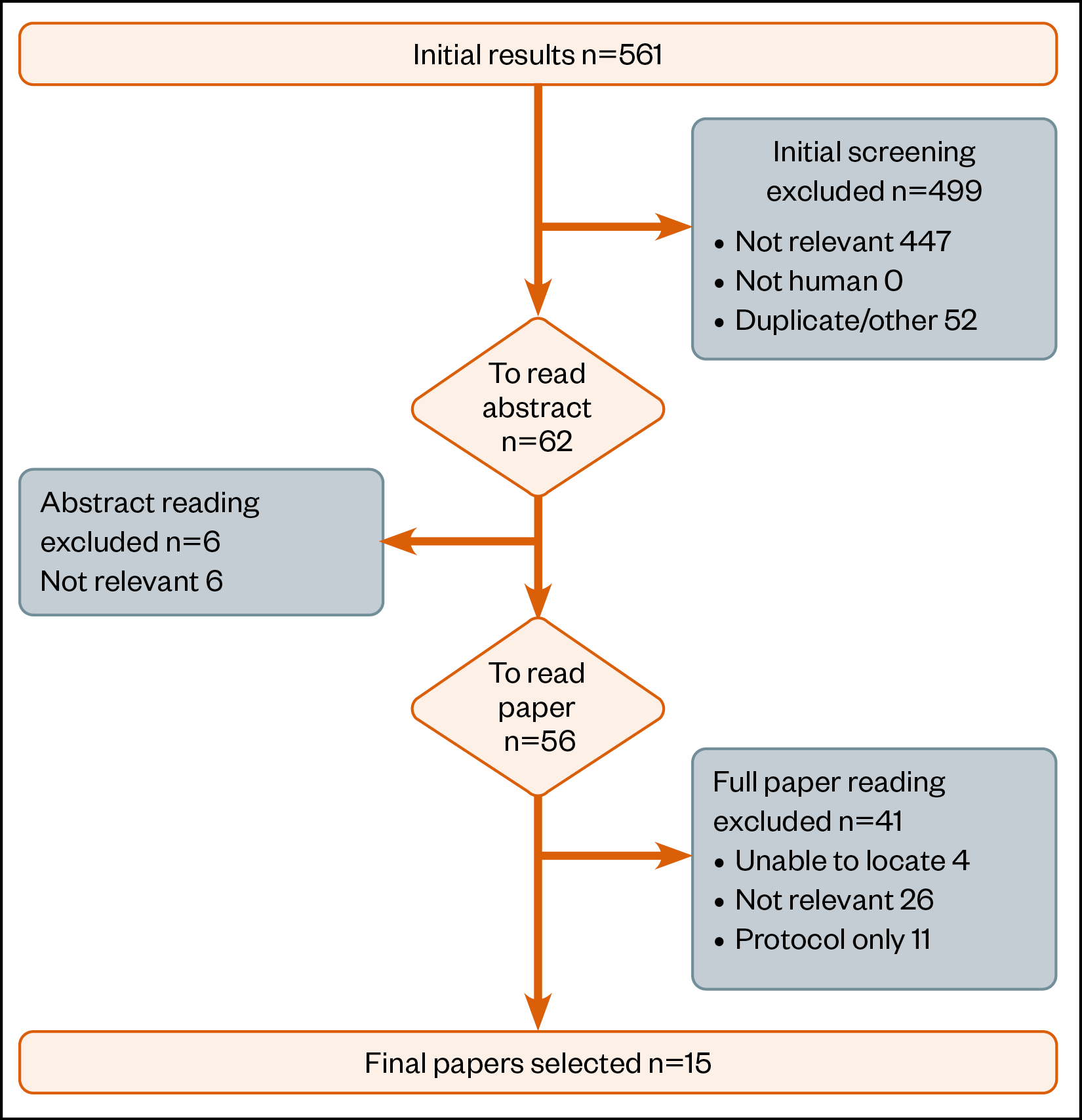
Table 1 provides an overview of the papers that were reviewed. PGx services in community pharmacies have been evaluated in the US, Canada and the Netherlands[10–19]. Within the US, Canada and Cyprus research has also been undertaken to identify what patients or pharmacists would need to either accept or deliver such a service[20–24].
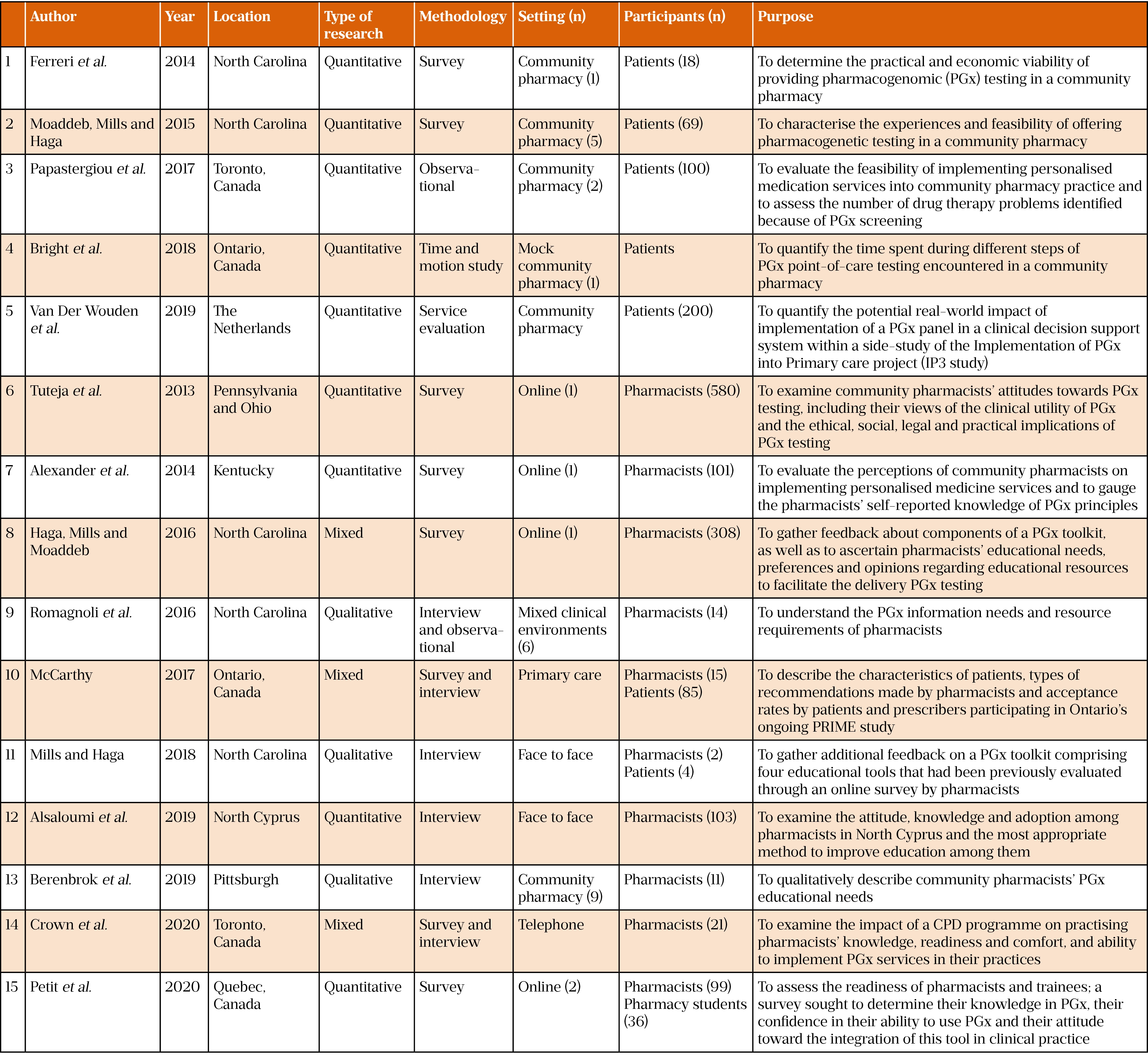
Five studies were designed for feasibility purposes[10,11,16,17,19], one study tested PGx test results recording[19], four papers reviewed pharmacist acceptability of a PGx testing service[20,21,23,24], one paper reviewed prescriber acceptability of the service[18], six papers reviewed pharmacist education needs[12,15,21–24], and three papers reviewed pharmacist information needs[12–14].
Enablers
Enablers for the implementation of a PGx testing service, which made pharmacists more likely to set up and implement the service, were identified and included:
- Patient interest in PGx testing and results;
- Pharmacist willingness and confidence to deliver the service;
- The service being comparable to existing pharmacy services;
- Prescriber acceptance of the results.
An important enabler was that patients were interested in their PGx results. In a 2014 study conducted by a pharmacist in a community pharmacy in North Carolina, United States, 62% of patients (n=18) were interested in learning more about the concept and a further 2014 study conducted across five community pharmacies, also in North Carolina, found that many patients were interested in PGx testing, with 81% (n=56) consenting to testing[10,11].
Community pharmacists’ positive perceptions were also an enabler. A 2017 study conducted in two busy urban community pharmacies in Toronto, Canada, found that community pharmacists have “the confidence and capability to successfully implement PGx into clinical practice” and, after receiving triage training, could identify patients who may benefit from this service[16]. Tujeta et al. found that, of 580 US community pharmacists, 87% (n=504) felt that PGx would improve medicine optimisation and reduce the occurrence of adverse events, while Alsaloumi et al. highlighted that community pharmacists “are well positioned to play an important role in the application of PGx in clinical practice”, as well as reporting that community pharmacists in North Cyprus see PGx testing as a vital part of their future role and held positive views about its clinical utility[20,24]. Encouragingly, 75% (n=76) of 101 independent community pharmacists in Kentucky, United States, surveyed were interested in integrating PGx into their clinical services portfolio[21]. Community pharmacists surveyed in Quebec, Canada, in 2018 (n=67) had a positive attitude overall to PGx and were willing to integrate it into their practice[23]. In one paper, 57% (n=319) of US pharmacists surveyed noted that it was their role to counsel patients following receipt of the PGx test results[20].
Another enabler is that PGx testing is no more onerous to implement than normal pharmacy services and utilises the full team to deliver the service. Moaddeb et al. reported that “the provision of PGx services appears feasible, requiring little additional time from the pharmacist”, while Bright et al. found that PGx testing is comparable to other existing community pharmacy services, such as a vaccination service[11,17]. The importance of engaging the entire pharmacy team was noted and, more specifically, that “pharmacy technicians could reduce the pharmacist hands-on time from 9.49 minutes to 2.64 minutes” for the delivery of the service[15,17].
Finally, several papers noted that good prescriber acceptance of pharmacists recommendations was an enabler, with Mills & Haga noting that “prescriber acceptance was exceptional for this new service”[10,18].
Barriers
Barriers to the implementation of a PGx testing service, which made the service less effective and the pharmacist less likely to set up and implement the service, were also identified and included:
- Inadequate education and training of pharmacists;
- Restricted access to appropriate clinical resources;
- Lack of patient-friendly materials;
- Lack of time capacity.
The training and education of pharmacists and prescribers was mentioned in several papers as being a barrier to implementation. Pharmacists recognised the shortfall in their education and, in one paper, 81% (n=74) of those surveyed said they would be willing to attend an appropriate training programme[15,20,21,24]. Specifically, training on interpreting test results and the communication of them to prescribers and patients may be required[11]. It was also suggested that pharmacist lack of knowledge and experience of the service may reduce the benefit of PGx testing for patients, and that pharmacists need further education on managing and discussing PGx test results[12,23]. Pharmacists “recognised their needs for enriched knowledge and instruction…with team-based approaches, robust clinical resources, and access to pharmacogenomic experts”, similar to immunisation-certified training programmes[15]. A potential shortfall in the knowledge of prescribers was also noted[16].
Having access to appropriate clinical resources was also reported as a barrier to implementation. This included clinical support systems, as pharmacists need full access to the patient’s medication record, with the need for an accurate record of the PGx results[10,19]. It was noted that “most community pharmacies are not equipped with the necessary resources to provide counselling regarding PGx” and that pharmacists require trustworthy PGx information to support their clinical decision making. Only “knowing the patient’s genotype is insufficient; knowing how to proceed is critical”[13,20].
A lack of appropriate, patient-friendly PGx resources was also reported as an important barrier. Haga et al. noted “the use of visual aids or graphics may help convey complex scientific or medical concepts” and that digital versions “enable patients to review the materials at home or share with family members”[12].
Finally, time capacity was reported as a barrier to implementation. Papastergiou et al. reported “it would be difficult to provide this time commitment in a traditional community pharmacy setting that focuses primarily on dispensing”, as the average initial consultation takes around 25 minutes[16].
Patient-orientated outcomes
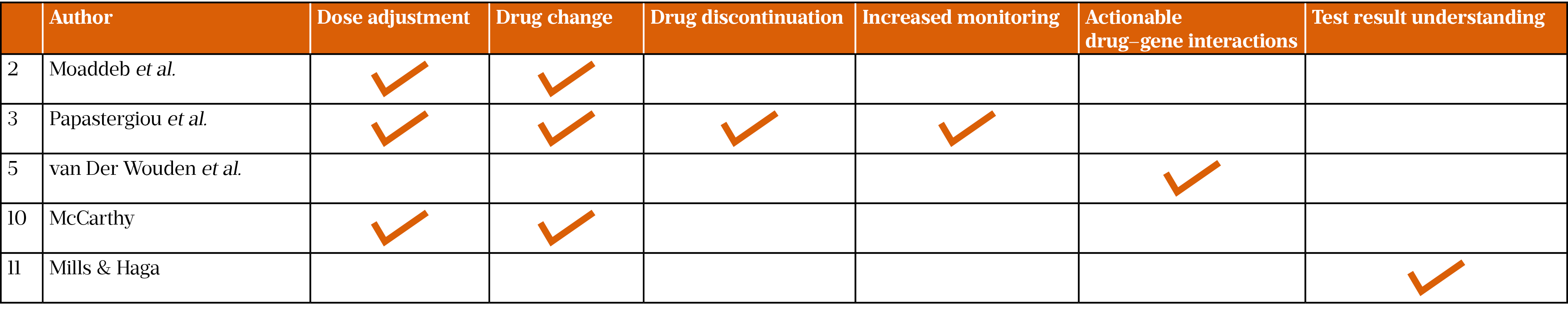
Common patient outcomes were reported in five papers: dose adjustment, change or discontinuation of medicine, increased monitoring of patient, actionable drug–gene interactions and understanding of test results by patients (Table 2)[11,14,16,18,19]. It was not established whether community pharmacy had a positive impact on patient outcomes; therefore, more research is required in this area. However, the research reported could have implications for the reduction of both adverse drug reactions and ‘trial-and-error’ prescribing.
Recommendations for future research
Suggestions and recommendations for future research were noted in some papers. These included how best to support the service and how to optimise related training and education programmes.
More research is required to investigate the best communication methods to use with prescribers to support the service, and understand the effect of PGx testing on existing pharmacy services, clinical outcomes, patient experience and satisfaction, as well as to assess “the most cost-effective approach regarding timing, target population, variants and techniques for PGx testing”[10,11,19].
To optimise training, further research is required into how to increase community pharmacists’ knowledge of PGx and tools to aid pharmacists in counselling patients[20]. In addition, research is needed to develop education programmes to prepare pharmacists to provide PGx services, including the availability and interpretation of PGx results, and evaluation of the training in a practice-based environment[14,21,24]. There was also a recommendation for “the additional integration of implementation science theory and approaches into curriculum design to address the challenges encountered by pharmacists as they incorporate this new service into their existing practices”[22].
Finally, while outside the scope of the study, it was noted in the reviewed papers that there was no direct evidence of the perception that GPs have of the service or of their acceptance of pharmacists’ recommendations to make changes to the prescription — there is therefore an opportunity for future research to evaluate this.
Discussion
PGx is a relatively novel area of medicine. The main finding of this scoping review is that there is little literature available regarding the experience of, and recommendations for, delivering a PGx testing service in a community pharmacy setting. However, despite the small number of published papers with empirical data, clear barriers to, and enablers of, implementation have been reported.
Patients, pharmacists and prescribers are the key stakeholders in PGx, and enablers related to each of them were noted. Patient interest in PGx as a concept and finding out about their own test results was of particular importance, as patients are, ultimately, the users of the service[10,11]. Pharmacists consider the delivery of the service to be their role, as evidenced by their willingness and confidence to identify patients, deliver the service and undergo further training[20,21,23,24]. This could be part of the natural evolution of the role of the community pharmacist, from dispensing medicines to providing an increasing number of clinical pharmacy services[25]. Feasibility was underlined by the fact that the service is comparable to existing community pharmacy services (e.g. the influenza vaccination service) and therefore not too onerous — an important capacity point in a busy pharmacy setting with increasing numbers of additional services being added to the portfolio[26]. Finally, pharmacists’ second-hand reporting of positive prescriber acceptance is an important element of implementation because, without their acceptance, the service would not function[10,18]. However, there was the least evidence of this. The advent of primary care networks in England will also support this system-wide collaboration between all healthcare professionals[27].
By contrast, the identified barriers mean that some environmental and educational changes may be required[12,15,16,20,21,23,24]. The appropriate education and training of pharmacists will be important in ensuring that pharmacists and their teams are competent and confident to deliver this service. The Centre for Pharmacy Postgraduate Education and Health Education England have produced some new training materials, which introduce the concepts of genomics in pharmacy, but specific guidance on setting up a PGx testing service in a community pharmacy setting in England is also required[28,29]. The General Pharmaceutical Council published new initial education and training standards that include education on PGx in 2021 [30].
Having access to appropriate clinical resources, including full patient medication records, is essential to make a full assessment of patient’s test results; this is now available through pharmacists’ full (but read-only) access to patients’ summary care records[30]. Appropriate patient-friendly resources to support the consultation need to be developed.
The right physical environment in the form of a professional consultation room, suitably sized and equipped for buccal swab testing, is also essential. Over recent years, most community pharmacies in England have installed such a facility and many owners have invested in both upgraded and additional rooms to a high standard[31]. Finally, the importance of having a good support team, including pharmacy technicians, and time to deliver the service should never be underestimated for the successful delivery of any clinical service in a community pharmacy setting.
The reported patient outcomes of dose adjustment, change or discontinuation of medicine and increased monitoring of patients are significant in improving outcomes for patients and reducing costs for the NHS[11,16,18]. Although the evidence for drug-gene pairs is of a similar quality as that available for drug–drug interactions, advice relating to them is generated using international databases by expert panels to determine drug-gene pairs that have clinical utility. Quality assurance is an important part of the process and most models involve sending DNA samples to a centralised service. Cost-effectiveness has been established for a number of drug–gene pairs and the cost of testing is reducing. Furthermore, the scale of the opportunity was noted to be significant, with a total of 24.2% of prescriptions having potential actionable drug–gene interactions, requiring some pharmacotherapy adjustment[19].
Limitations of the review
As a scoping review, with limited use of databases and search terms, there may be papers missing, meaning the data may not accurately reflect the knowledge regarding the topic within the literature. However, our results suggest that a systematic review is warranted. Only one researcher in the team developed the barrier and enabler themes, but the research team believes that they coherently characterise the relevant literature. There was a limited sample size for some of the studies and none of the studies were undertaken in the UK; therefore, the findings may not be applicable to community pharmacy in the UK.
Implications for practice
There is an opportunity for the implementation of a PGx testing service in community pharmacies in the UK. However, there is a clear need to educate the profession and to consider whether training should be included in undergraduate programmes or provided when pharmacists begin offering the service.
Developing clinical support materials and linking them to the patient’s summary care record will be an important consideration; this will need to be developed in conjunction with the PGx testing providers. Translating this data into information that is easy to understand for the patient is essential and consideration needs to be given to either a paper-based or digital format for their personal summary report.
Finally, the pharmacist–GP interface is important, both in terms of appropriate communication and GPs themselves understanding how to interpret test results and the implications for prescribing changes. In addition, the interplay between other professions, including genomics laboratories and secondary care teams, needs consideration. Helpfully, the advent of independent prescribers in community pharmacy will allow pharmacists to make changes to medications and doses in the future — in collaboration with GPs, but without having to seek authorisation.
Conclusion
There is an opportunity for a PGx testing service in community pharmacy to make a difference to patients’ lives. The evidence in this review indicates that patients would welcome the opportunity, that pharmacists in other countries see this as part of their role and that GPs will be supportive and collaborative (although there was no first-hand evidence of this in the results). To successfully implement the service, pharmacists and their teams will require training, and will need to be supported by robust clinical support systems and appropriate and relevant patient information for face-to-face consultations. Effective communication with GPs is also vital. It is essential that all barriers — physical and behavioural — are considered and addressed as part of any design of a PGx testing service before implementation. Community pharmacists have developed their role in recent years to offer more clinical services, including medication reviews, healthy-living advice and vaccination services. The introduction of PGx testing could be a further evolution of this model and a significant boost to the pharmacist’s role as the expert in medicines management.
Key points:
- Pharmacogenomics (PGx) is the use of DNA to predict an individual’s response to drugs;
- The outcome from PGx testing is better drug and safer dose selection, improved patient care and lower costs to the NHS;
- PGx testing has been introduced in community pharmacy in some parts of the world, but not yet in the UK;
- Enablers to implementation include patient interest in PGx, pharmacist willingness and confidence to deliver the service and undergo further training, the service being comparable to existing pharmacy services, and prescriber acceptance of the results;
- Barriers to implementation include the need for pharmacist training, restricted access to appropriate clinical resources, lack of patient friendly resources and time capacity;
- There is an opportunity to build on the enablers and overcome the barriers to design a PGx testing service for community pharmacy in the UK.
Financial disclosure and conflict of interest statement
Tim Rendell is head of pharmacy at Day Lewis PLC. He is also a professional doctorate student at the University of Bath, studying pharmacogenomics, and chair of the Operations Board at the Centre for Pharmacy Postgraduate Education, based at the University of Manchester.
David Wright has a research collaboration with myDNA Life Australia but has no financial interest in the company.
- 1Prescriptions dispensed in the community — statistics for England, 2007–2017 . NHS Digital. 2017.https://digital.nhs.uk/data-and-information/publications/statistical/prescriptions-dispensed-in-the-community/prescriptions-dispensed-in-the-community-england—2007—2017 (accessed Nov 2021).
- 2Leucht S, Hierl S, Kissling W, et al. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta-analyses. Br J Psychiatry. 2012;200:97–106. doi:10.1192/bjp.bp.111.096594
- 3Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi:10.1136/bmj.329.7456.15
- 4Vogenberg F, Isaacson B, Pursel M. Personalized medicine: part 1: evolution and development into theranostics. P T 2010;35:560–76.https://www.ncbi.nlm.nih.gov/pubmed/21037908
- 5Pirmohamed M. Personalized Pharmacogenomics: Predicting Efficacy and Adverse Drug Reactions. Annu. Rev. Genom. Hum. Genet. 2014;15:349–70. doi:10.1146/annurev-genom-090413-025419
- 6The accessibility of community pharmacies. Pharmaceutical Services Negotiating Committee. 2019.https://psnc.org.uk/psncs-work/about-community-pharmacy/ (accessed Nov 2021).
- 7NHS Genomic Medicine Service. NHS England. 2020.https://www.england.nhs.uk/genomics/nhs-genomic-med-service/ (accessed Nov 2021).
- 8Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8:19–32. doi:10.1080/1364557032000119616
- 9Lima DKS, Schoeller SD, Knihs N da S, et al. Protocol for a scoping review of skin self-care of people with spinal cord injury. BMJ Open. 2017;7:e017860. doi:10.1136/bmjopen-2017-017860
- 10Ferreri SP, Greco AJ, Michaels NM, et al. Implementation of a pharmacogenomics service in a community pharmacy. Journal of the American Pharmacists Association. 2014;54:172–80. doi:10.1331/japha.2014.13033
- 11Moaddeb J, Mills R, Haga SB. Community pharmacists’ experience with pharmacogenetic testing. Journal of the American Pharmacists Association. 2015;55:587–94. doi:10.1331/japha.2015.15017
- 12Haga SB, Mills R, Moaddeb J. Evaluation of a pharmacogenetic educational toolkit for community pharmacists. Pharmacogenomics. 2016;17:1491–502. doi:10.2217/pgs-2016-0002
- 13Romagnoli KM, Boyce RD, Empey PE, et al. Bringing clinical pharmacogenomics information to pharmacists: A qualitative study of information needs and resource requirements. International Journal of Medical Informatics. 2016;86:54–61. doi:10.1016/j.ijmedinf.2015.11.015
- 14Mills R, Haga SB. Qualitative user evaluation of a revised pharmacogenetic educational toolkit. PGPM. 2018;Volume 11:139–46. doi:10.2147/pgpm.s169648
- 15Berenbrok LA, Hart KM, McGrath SH, et al. Community pharmacists’ educational needs for implementing clinical pharmacogenomic services. Journal of the American Pharmacists Association. 2019;59:539–44. doi:10.1016/j.japh.2019.03.005
- 16Papastergiou J, Tolios P, Li W, et al. The Innovative Canadian Pharmacogenomic Screening Initiative in Community Pharmacy (ICANPIC) study. Journal of the American Pharmacists Association. 2017;57:624–9. doi:10.1016/j.japh.2017.05.006
- 17Bright DR, Klepser ME, Murry L, et al. Pharmacist-Provided Pharmacogenetic Point-of-Care Testing Consultation Service: A Time and Motion Study. Journal of Pharmacy Technology. 2018;34:139–43. doi:10.1177/8755122518756651
- 18Pharmacy Practice Research Abstracts: Canadian Pharmacists Conference 2017. Can Pharm J. 2017;150:S1–72. doi:10.1177/1715163517719855
- 19van der Wouden CH, Bank PCD, Özokcu K, et al. Pharmacist-Initiated Pre-Emptive Pharmacogenetic Panel Testing with Clinical Decision Support in Primary Care: Record of PGx Results and Real-World Impact. Genes. 2019;10:416. doi:10.3390/genes10060416
- 20Tuteja S, Haynes K, Zayac C, et al. Community pharmacists‘ attitudes towards clinical utility and ethical implications of pharmacogenetic testing. Personalized Medicine. 2013;10:793–800. doi:10.2217/pme.13.85
- 21Alexander KM, Divine HS, Hanna CR, et al. Implementation of personalized medicine services in community pharmacies: Perceptions of independent community pharmacists. Journal of the American Pharmacists Association. 2014;54:510–7. doi:10.1331/japha.2014.13041
- 22Crown N, Sproule BA, Luke MJ, et al. A Continuing Professional Development Program for Pharmacists Implementing Pharmacogenomics into Practice. Pharmacy. 2020;8:55. doi:10.3390/pharmacy8020055
- 23Petit C, Croisetière A, Chen F, et al. Are pharmacists from the province of Quebec ready to integrate pharmacogenetics into their practice. Pharmacogenomics. 2020;21:247–56. doi:10.2217/pgs-2019-0144
- 24Alsaloumi L, Abdi A, Tosun Ö, et al. Pharmacogenomics-based practice in North Cyprus: its adoption by pharmacists and their attitudes and knowledge. Int J Clin Pharm. 2019;41:1299–306. doi:10.1007/s11096-019-00868-6
- 25Community Pharmacy Clinical Services Review. NHS England. 2016.https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2016/12/community-pharm-clncl-serv-rev.pdf (accessed Nov 2021).
- 26Services and commissioning. Pharmaceutical Services Negotiating Committee. 2021.https://psnc.org.uk/services-commissioning/ (accessed Nov 2021).
- 27Primary care networks. NHS England. 2021.https://www.england.nhs.uk/primary-care/primary-care-networks/ (accessed Nov 2021).
- 28Introduction to genomics in pharmacy. Centre for Pharmacy Postgraduate Education. 2020.https://www.cppe.ac.uk/programmes/l/genomics-e-01/ (accessed Nov 2021).
- 29Pharmacogenomics and stratified healthcare. Health Education England. 2021.https://www.genomicseducation.hee.nhs.uk/education/taught-courses/pharmacogenomics-and-stratified-healthcare/ (accessed Nov 2021).
- 30Benefits and uses of Summary Care Records in community pharmacy. NHS Digital. 2020.https://digital.nhs.uk/services/summary-care-records-scr/summary-care-record-scr-in-community-pharmacy/benefits-and-uses-of-scr-in-community-pharmacy (accessed Nov 2021).
- 31How your pharmacy can help. NHS. 2019.https://www.nhs.uk/nhs-services/prescriptions-and-pharmacies/pharmacies/how-your-pharmacy-can-help/ (accessed Nov 2021).