CYB003

CYB003: Deuterated Psilocybin Analog with FDA Breakthrough Therapy Designation
CYB003 is a proprietary deuterated molecule related to psilocybin, which is part of a family of molecules called indolamines that includes more common neurotransmitters like serotonin. CYB003 is a new chemical entity (NCE) and has been granted FDA Breakthrough Therapy Designation for the adjunctive treatment of Major Depressive Disorder (MDD).
Development Status: Completed Phase 2 trial of CYB003 in participants with moderate to severe MDD
Next Steps: Initiate Phase 3 pivotal, multinational trial of CYB003 in MDD in mid-2024
CYB003 received the first known Breakthrough Therapy Designation for an adjunctive psychedelic-based therapy for MDD
This designation acknowledges the significant unmet medical need for more effective treatment of MDD. It also provides validation that Phase 2 data for CYB003 shows preliminary evidence for significant clinical improvements over existing therapies.
Breakthrough Therapy Designation expedites the development pathway for CYB003
Breakthrough Therapy Designation benefits:
All Fast Track Designation features, including Rolling Review for NDA
More intensive FDA guidance and discussion, including planned clinical trials and plans for expediting manufacturing development strategy
CYB003 is eligible for Priority Review and Accelerated Approval

Clinical Validation for CYB003 in MDD: Robust and Sustained Remission Rates up to Four Months
To date, Cybin has announced positive Phase 2 topline results for CYB003 in participants with moderate to severe MDD, evaluating the efficacy and safety of CYB003 with two doses, at up to 4 months.
Improvement in symptoms after single dose
At 3 weeks:
12mg better than placebo on MADRS by 14.1 points (p=0.0001), Cohen’s d=2.31
16mg better than placebo on MADRS by 13 points (p=0.008), Cohen’s d=2.31
Average 5.8 points improvement on the MADRS after 2nd dose (12mg)
>75% response rates and up to 80% remissions rates (12mg) after a 2nd dose
Benefit sustained 4 months after 2nd dose with 60% of patients on 12mg and 75% of patients on 16mg in remission
Excellent safety and tolerability profile – all reported AEs mild to moderate; no AEs of suicidality
Phase 3 plan aligned with FDA at end of phase 2 meeting
Strong and Durable Effects 4 Months After 2 Doses of CYB003
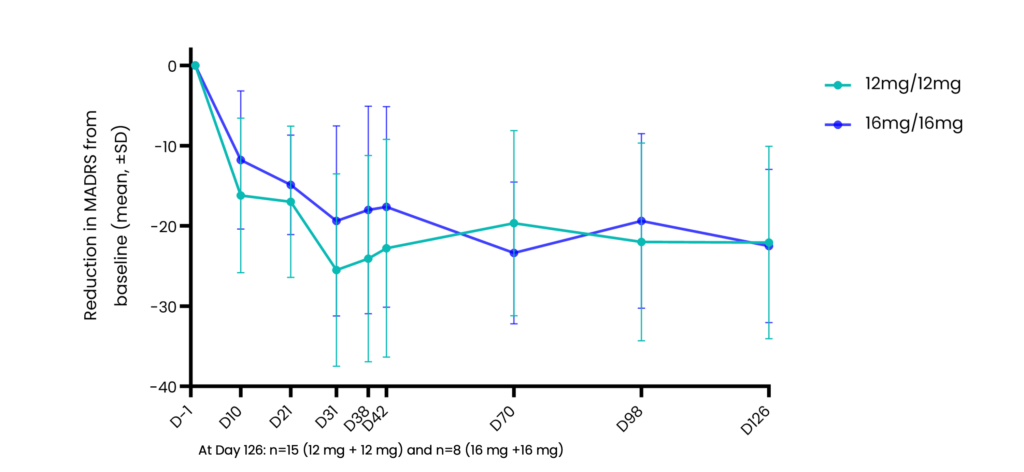
Mean 22-point reduction in MADRS scores from baseline at 4 months after just two doses of CYB003
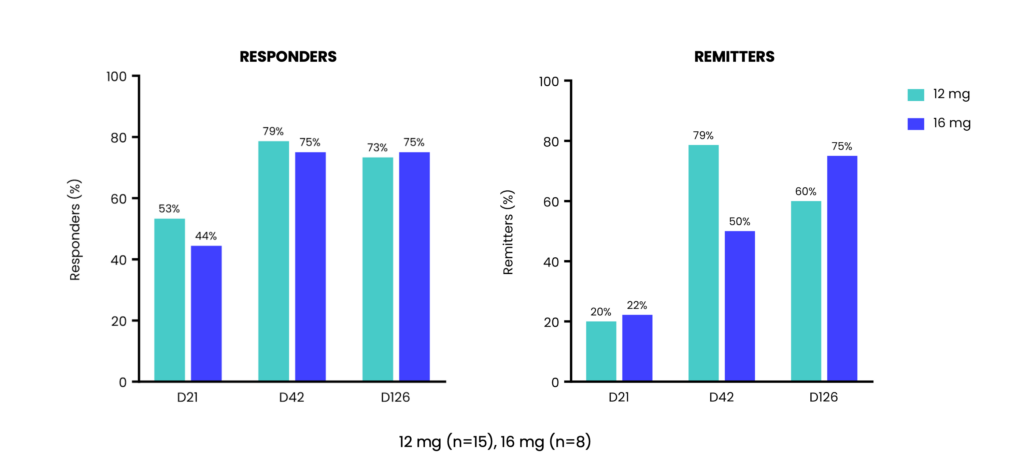
Sustained benefit 4 months after 2nd dose with 60% of patients on 12 mg and 75% on 16 mg in remission
Expanded Access Policy
We do not offer expanded access at this time.
Discover Cybin’s complete Research and Development Progress.



